Full-Form of DMSO In Chemistry
It is often used as a solvent in organic chemistry. It is an organosulfur compound with the chemical formula C2H6OS. The following is the DMSO structure. It has a sulfur atom at the center bounded by two methyl groups via single bonds and an oxygen atom joined via a double bond with the sulfur atom.
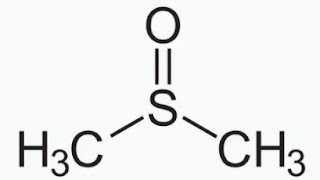 |
| DMSO Structure |
The solvent is a colorless liquid and it
dissolves in both polar and nonpolar chemical compounds.
The
solvent is miscible in the following compounds
|
Acetic
Acid |
Butanol |
Carbon
Tetrachloride |
|
Acetone |
Methyl
ethyl ketone |
Chloroform |
|
Acetonitrile |
Butyl
Acetate (n-) |
Dichloromethane |
|
Benzene |
Methyl
tert-butyl ether |
Diisopropyl
ether |
|
Dimethylformamide |
1-Propanol |
Trichloroethylene |
|
Dioxane |
Isopropanol |
Toluene |
|
Ethanol |
Tetrahydrofuran |
Water |
|
Methanol |
Ethyl
Acetate |
Dichloroethane
(1, 2) |
The
solvent is miscible in the following compounds
|
Cyclohexane |
Diethyl
ether |
Heptane |
|
Hexane |
Pentane |
Isooctane |
|
Xylene |
|
|
Uses
of DMSO
·
DMSO
is commonly used as
·
Industrial
solvent
·
Solvent
for NMR
·
Reagent
in organic synthesis
·
Cryopreserve
and store cultured cells
·
Antifreezing
agent or hydraulic fluid when mixed with water
Industries that use DMSO
·
Polymer
·
Pharmaceutical
·
Agrochemicals
I
hope this solved your query what is the full form of DMSO, properties, and uses